Download Electron And Molecular Geometry Of H2O most complete GM
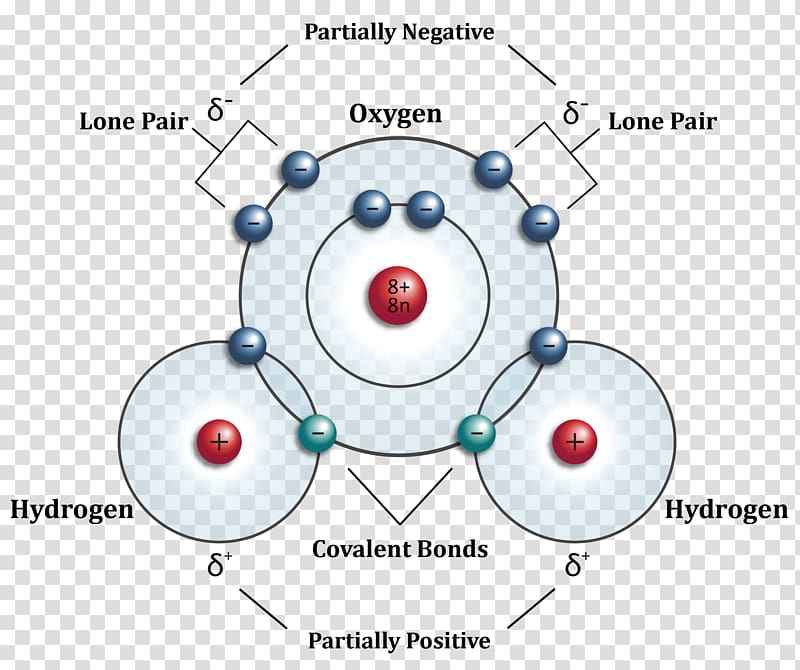
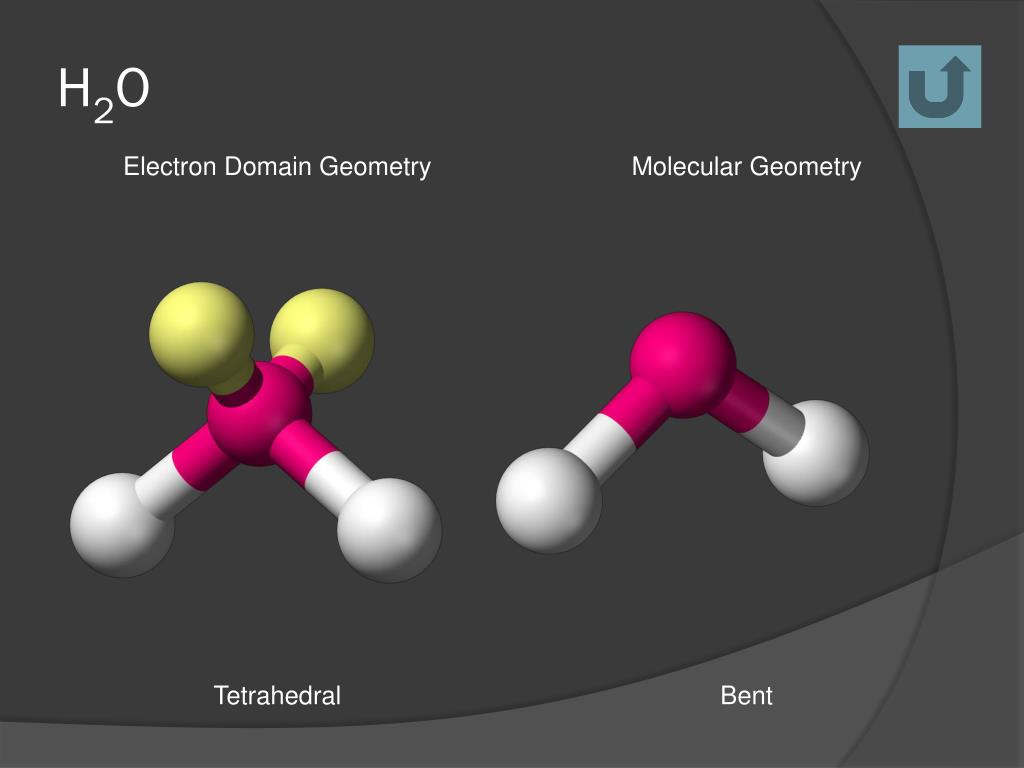
Examples: In a water molecule, H 2 O two of the central oxygen atom's valence electrons form two bond pairs with the hydrogen atoms, while the remaining four electrons form two lone pairs. Therefore, the molecular geometry of water is bent and the electron geometry of water is tetrahedral. Ammonia, NH 3, is another example with different molecular and electron geometries.
Hydrogen atom Water Molecule Molecular orbital diagram, water
Chapter 20 Chapter 21 Index By the end of this section, you will be able to: Predict the structures of small molecules using valence shell electron pair repulsion (VSEPR) theory Explain the concepts of polar covalent bonds and molecular polarity Assess the polarity of a molecule based on its bonding and structure
PPT Molecular Geometry PowerPoint Presentation, free download ID
Figure 10.2.2 ): (CC BY-NC-SA; anonymous) The two oxygens are double bonded to the sulfur. The oxygens have 2 lone pairs while sulfur had one lone pair. 3. There are two bonding pairs and one lone pair, so the structure is designated as AX 2 E. This designation has a total of three electron pairs, two X and one E.
Chemical structure of a water molecule, H2O Stock Photo Alamy
Figure 7.6. 9: (a) H 2 O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. (b) Two of the electron regions are lone pairs, so the molecular structure is bent. Exercise 7.6. 3. The hydronium ion, H 3 O +, forms when acids are dissolved in water.

Modelo De H2o
The Shapes of Molecules. The chemical bonding in a compound is very obviously related to its reactivity and properties - Na2O and H2O being quite different materials. It is perhaps less obvious that the shape of a molecule may also be crucial to its physical and chemical properties. sugar) yet gives a sweat sensation in the mouth.

H2O Lewis Structure, Molecular Geometry, and Hybridization
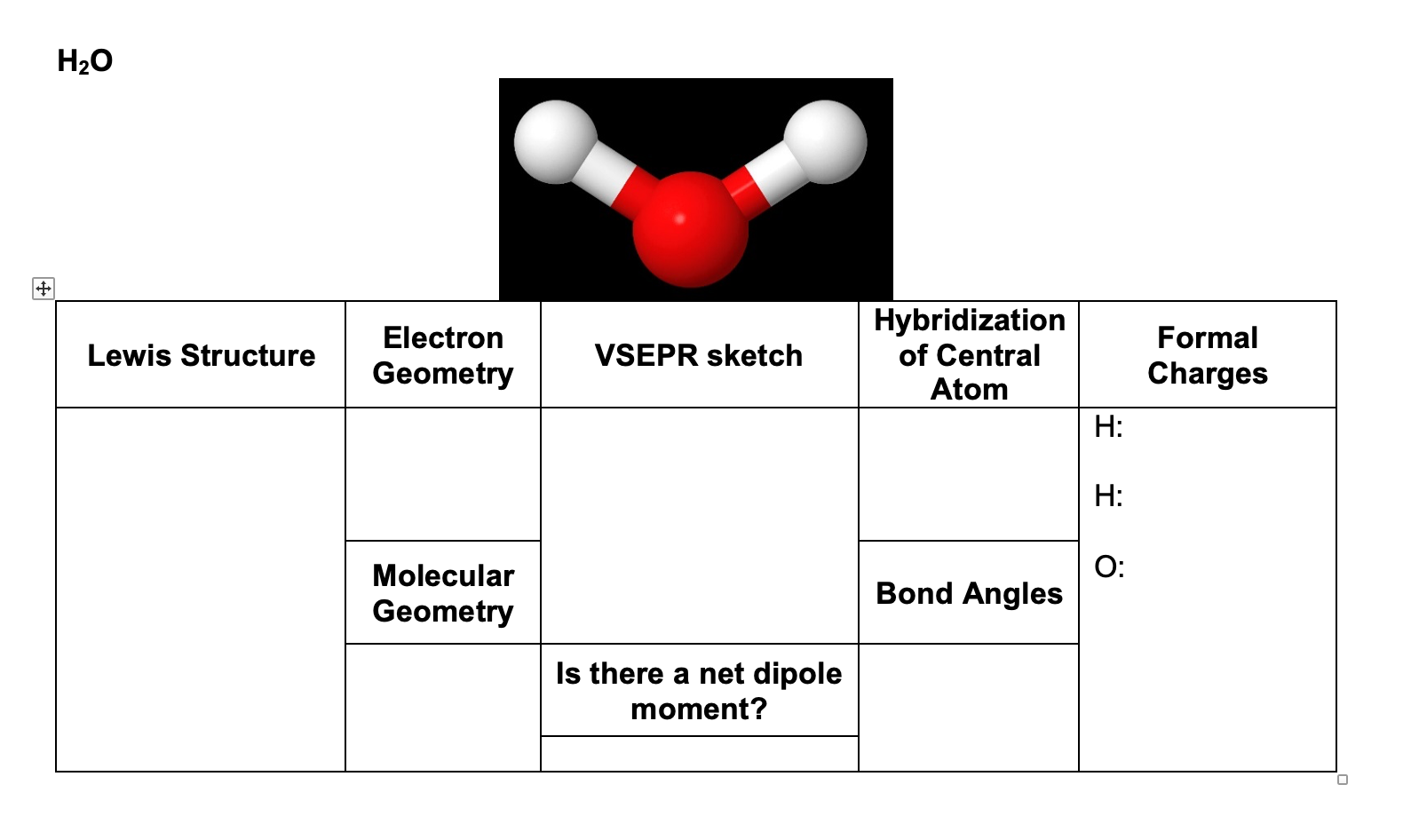
The predicted electron geometry of the H2O molecule is tetrahedral based on the VSEPR theory, which takes into account the four electron pairs surrounding the oxygen atom. This prediction is consistent with the observed tetrahedral electron geometry of the H2O molecule. The two lone pairs and two bond pairs of electrons occupy the four hybrid.
Solved H20 + Lewis Structure Electron Geometry VSEPR sketch
The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound.
Lewis Structure Of Water
As stated above, molecular geometry and electron-group geometry are the same when there are no lone pairs. The VSEPR notation for these molecules are AX n. "A" represents the central atom and n represents the number of bonds with the central atom. When lone pairs are present, the letter E x is added. The x represents the number of lone pairs.

H2O Molecular Geometry / Shape and Bond Angles YouTube
Electron Geometry for Water (H2O) The Lewis structure of hydrogen and 2 oxygen atoms shows a total of eight valence electrons participate in the bond formation to form a single triatomic H2O molecule. Here, we need to understand how the Lewis structure is drawn for the H2O molecule:

H20 Molecular Orbital Diagram
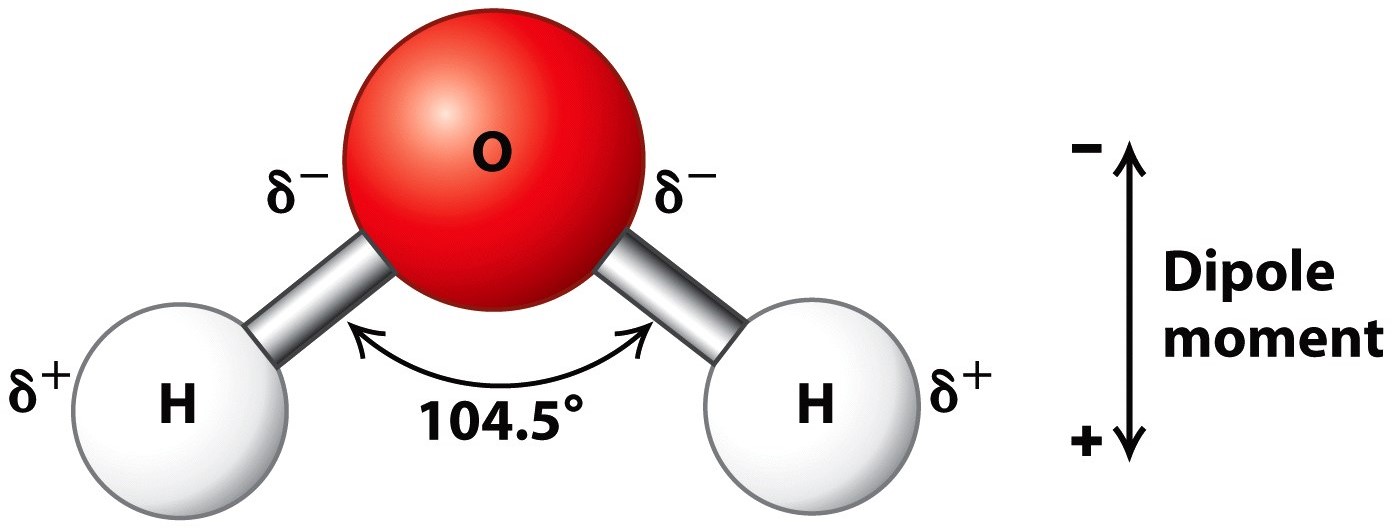
Around each oxygen atom there are 2 lone pairs, and 2 bonding pairs of electrons to form the O −H bonds. ∠H −O −H is compressed from the ideal tetrahedral angle of 109.5 ∘ to approx. 104.5 ∘ because the lone pairs are larger and more diffuse than a localized bonding pair, and therefore more likely to influence structure.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation
Chemistry Chemistry questions and answers Part B What is the electron geometry of H2O? Enter the electron geometry of the molecule. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Part B What is the electron geometry of H2O?

H2S Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
1. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. In the periodic table- Hydrogen is a Group IA element with one electron in its outermost shell (valence shell). Oxygen is a Group VIA element with six electrons in its last shell.

H2O. Molécule d'eau modèle, formule chimique, ballandstick modèle
A quick explanation of the molecular geometry of H2O (Water) including a description of the H2O bond angles..more.more Lewis Structures, Introduction, Formal Charge, Molecular.

Draw The Lewis Structure Of H2o Fotodtp
The angles between electron domains are determined primarily by the electronic geometry (e.g., 109.5° for a steric number of 4, which implies that the electronic shape is a tetrahedron). For example, the H-N-H bond angle in ammonia is 107°, and the H-O-H angle in water is 104.5°. We can rationalize this in terms of the last rule above.

H2O Lewis Structure, Molecular Geometry, and Hybridization
Figure 8.6.1 8.6. 1 shows the various molecular geometries for the five VESPR electronic geometries with 2 to 6 electron domains. When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation
What is the electron geometry and molecular geometry of H2O?
