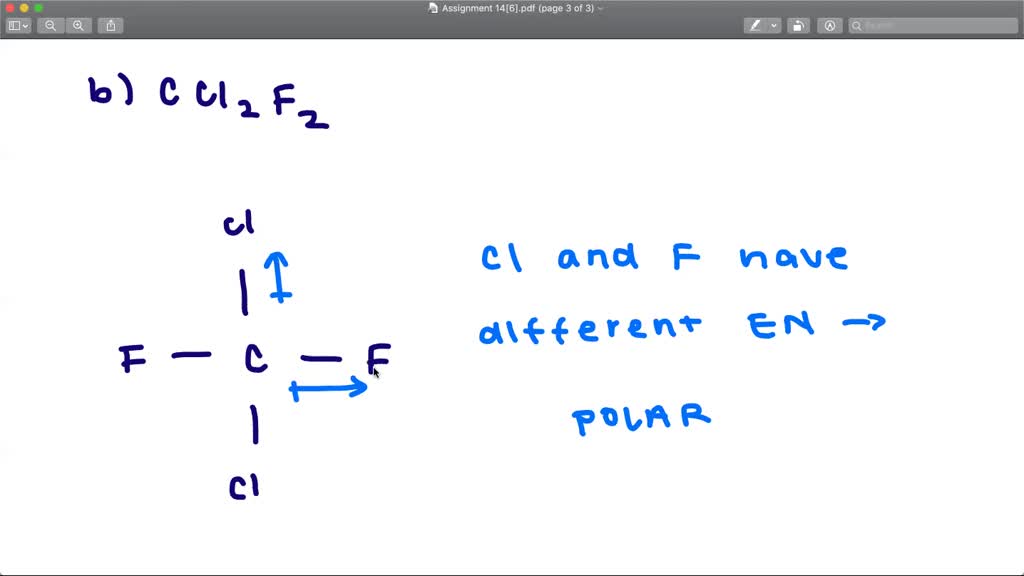
SOLVEDDetermine whether each molecule is polar or nonpolar. a. SiCl4 b
Polar or non-polar SiCl4 is a non-polar molecule. It comprises one silicon (Si) atom and four chlorine (Cl) atoms. The silicon is kept at the central position while all four chlorine atoms occupy surrounding positions. Specifically, the Lewis dot structure of SiCl4 shows four Si-Cl bonds. All four valence electrons of silicon used in covalent.
How To Know If A Molecule Is Polar Or Nonpolar Khan Academy
Silicon tetrachloride (SiCl4) is a non-polar molecule. It consists of one silicon (Si) atom and four chlorine (Cl) atoms. The silicon is kept at the central position while all four chlorine atoms occupy surrounding positions, making a perfectly symmetrical tetrahedral molecular shape and geometry.

SOLVEDDetermine whether each molecule is polar or nonpolar. a. SiCl4 b
When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.

Reading Covalent Bonds Biology I
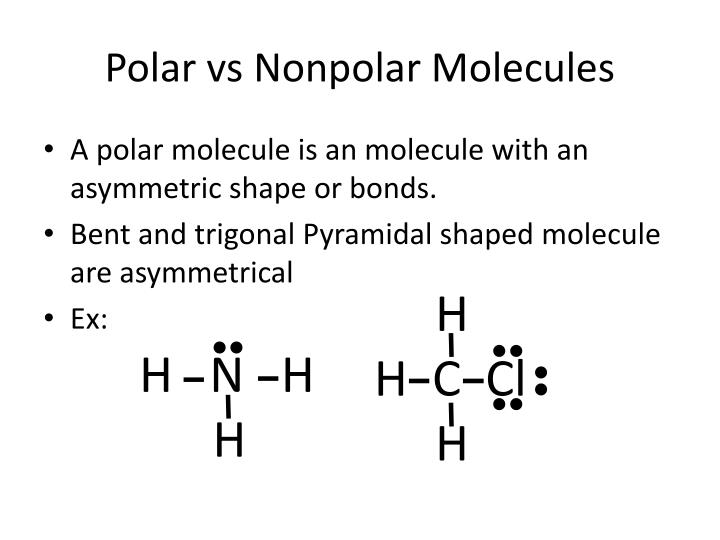
1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.12.1 4.12. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ). Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom.

Is SiCl4 Polar or NonPolar? YouTube
SCl4 is a POLAR molecule because the Chlorine (Cl) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire SCl4 molecule polar.

SiCl4 Lewis Structure (Silicon Tetrachloride) YouTube
SiCl4 is a NONPOLAR molecule because all the four bonds (Si-Cl bonds) are identical and SiCl4 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of SiCl4 lewis structure and its 3D geometry. Why is SiCl4 a Nonpolar molecule? (Explained in 3 Steps)
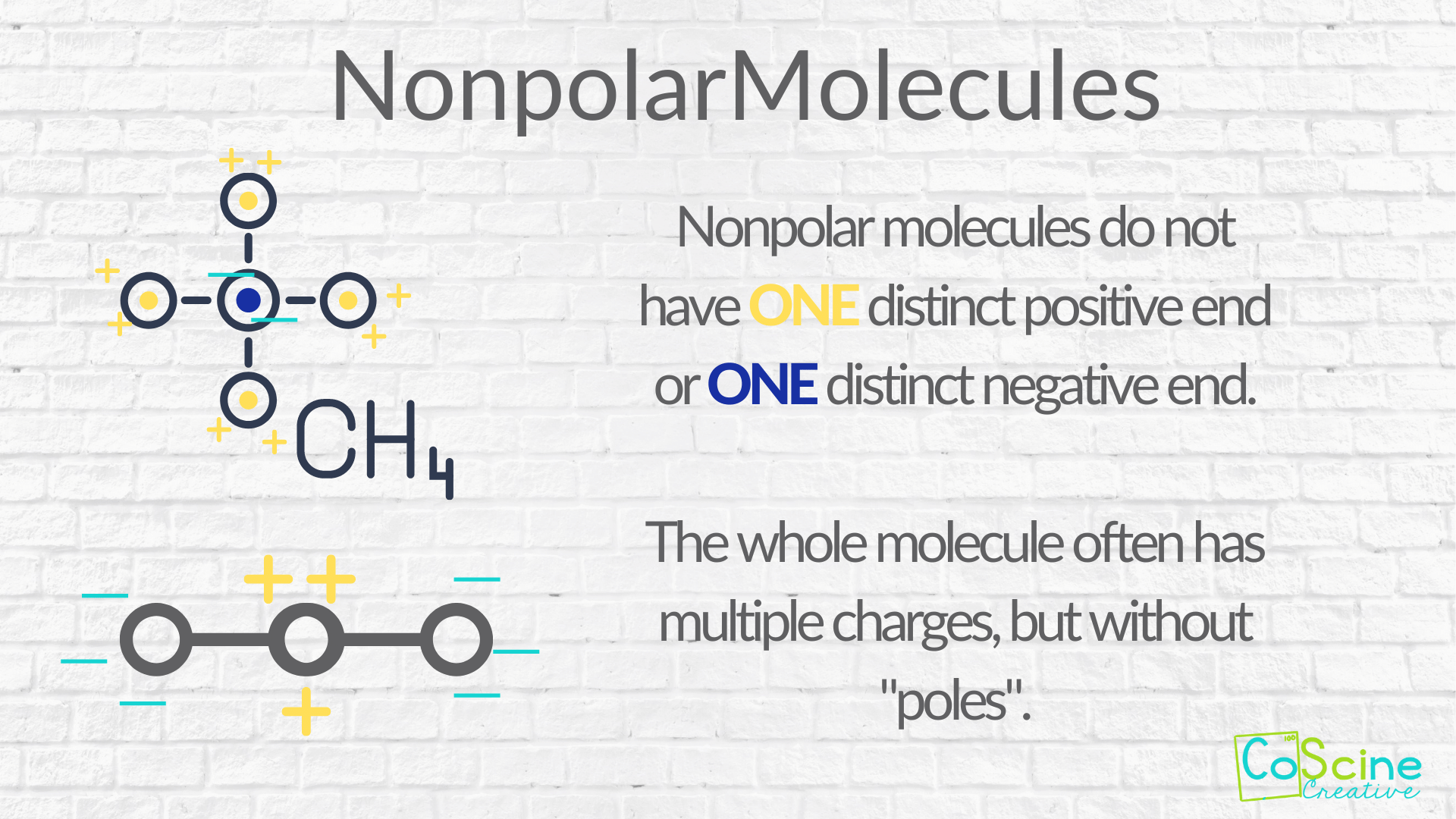
How Do You Teach Polar Vs. Nonpolar Molecules? — CoScine Creative
a. SiCl4 b.. | Channels for Pearson+ My Course Learn with Jules Exam Prep Explore Bookmarks Next problem 7:17 minutes Problem 52 Tro - 4th Edition Textbook Question Determine whether each molecule is polar or nonpolar. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5 Verified Solution

Is Scl4 Polar Or Nonpolar Facilities for the Public
Is Sicl4 polar or nonpolar? SiCl4 (silicon tetrachloride) is a nonpolar chemical. Because the four chemical bonds between silicon and chlorine are uniformly distributed, SiCl4 is non-polar. A polar covalent bond is a type of covalent link that is intermediate between pure covalent bonds and ionic bonds. When the difference in electronegativity.

Difference between polar and nonpolar examples
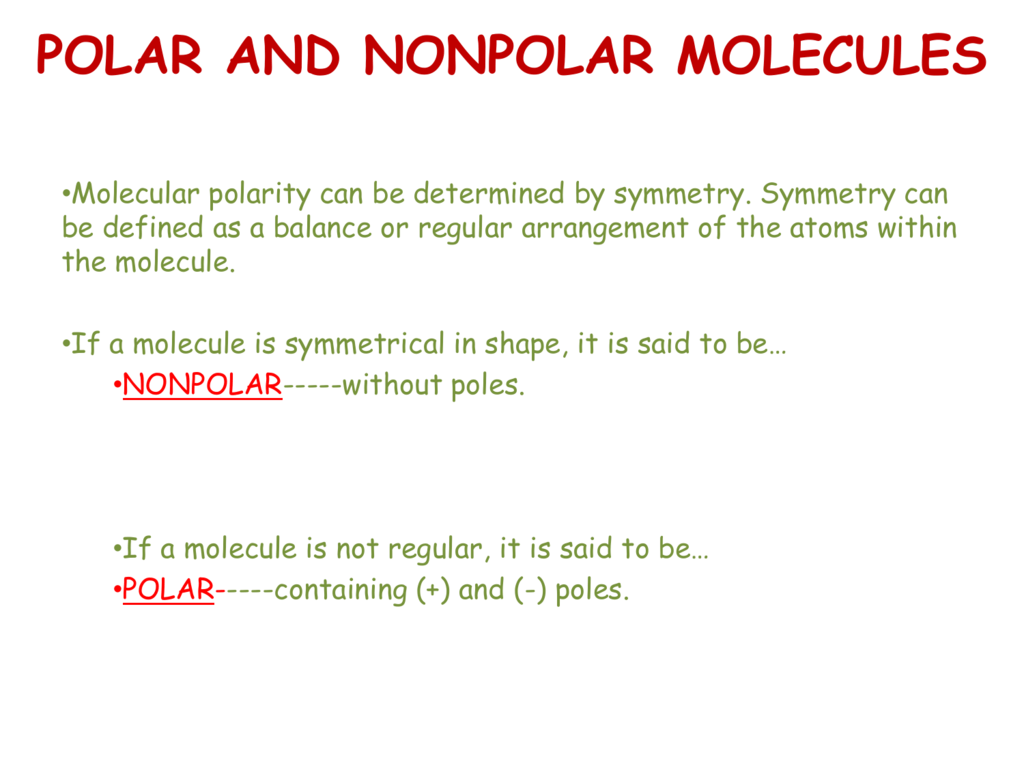
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric. For molecules with four or fewer total electron groups around the central atom, a symmetrical molecule is identical on all sides - the bonded atoms are identical and there are no unshared electrons on the central atom.

Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding
Bond Polarity Calculator Calculate the molecular polarity (polar, non-polar) of a chemical bond based on the electronegativity of the elements. First Element Second Element Calculate Bond Polarity How To Calculate Bond Type and Polarity The bond polarity between two atoms can be estimated if you know the electronegativity of both elements.
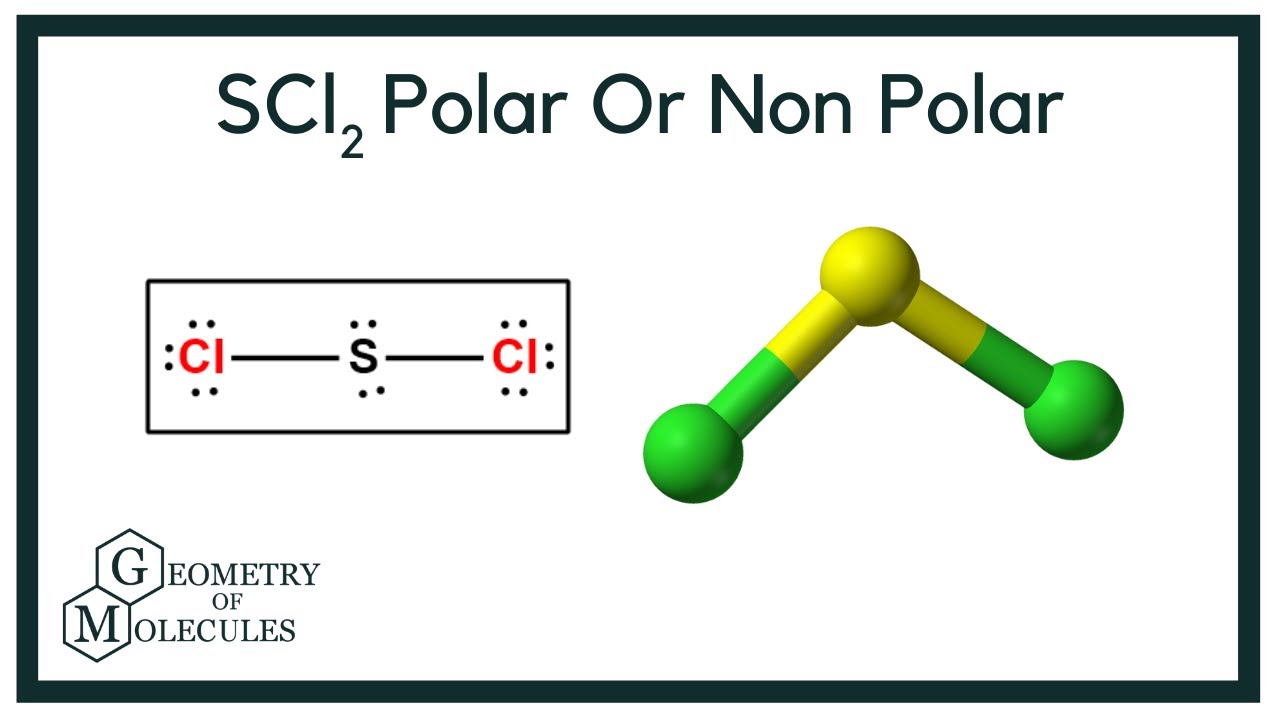
Is SCl2 Polar or NonPolar (Sulfur Dichloride) YouTube
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.

Polar and Nonpolar Molecules
Is SCl4 polar or non polar? Updated: 8/11/2023 Wiki User ∙ 15y ago Study now See answers (5) Best Answer Copy SCl4 is a polar molecule because when the shape is taken into consideration it is.
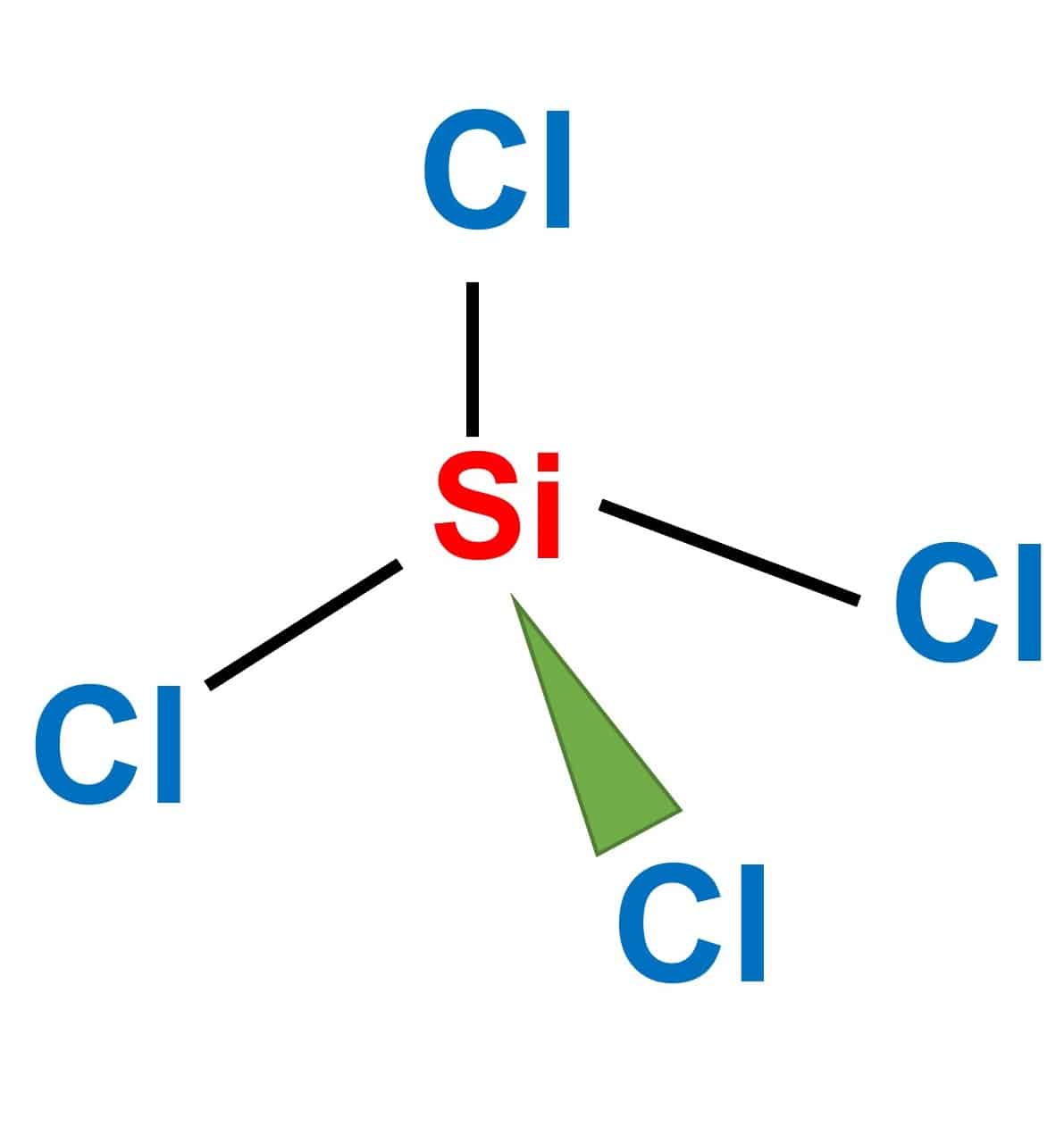
SiCl4 Polar or Nonpolar Easy Explanation What's Insight
SiCl4 (Silicon tetrachloride) is a non-polar molecule. SiCl4 is non-polar because the four chemical bonds between silicon and chlorine are equally distributed. Keep reading to know more about the question "SiCl4 polar or nonpolar".
PPT Polar or Nonpolar PowerPoint Presentation ID3483667
Learn to determine if SiCl4 (Silicon tetrachloride) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the L.
Is Sicl4 Polar Or Nonpolar
SeCl4 is a POLAR molecule because the Chlorine (Cl) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire SeCl4 molecule polar.

Polar and Nonpolar Covalent Bonds Characteristics & Differences
SiCl4 is silicon tetrachloride, which is a non-polar molecule. Silicon tetrachloride is non-polar because the four chemical bonds between silicon and chlorine are equally distributed. The even distribution of polar bonds results in a molecule that does not have distinct poles.
