
CS2 Molecular Geometry / Shape and Bond Angles YouTube
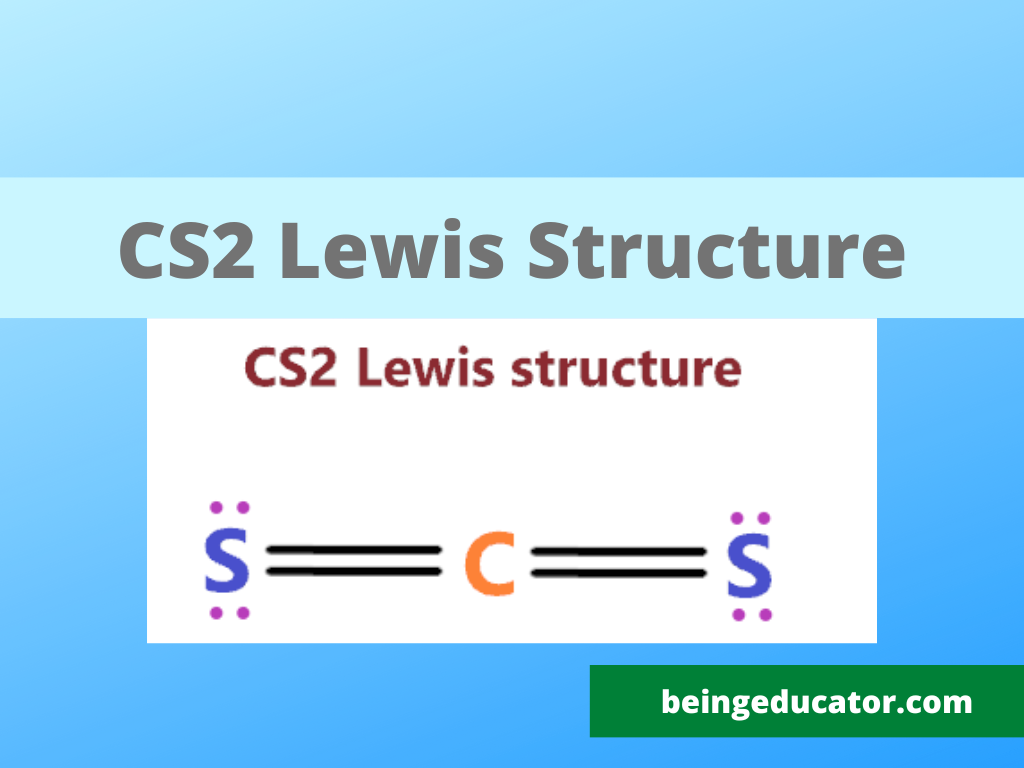
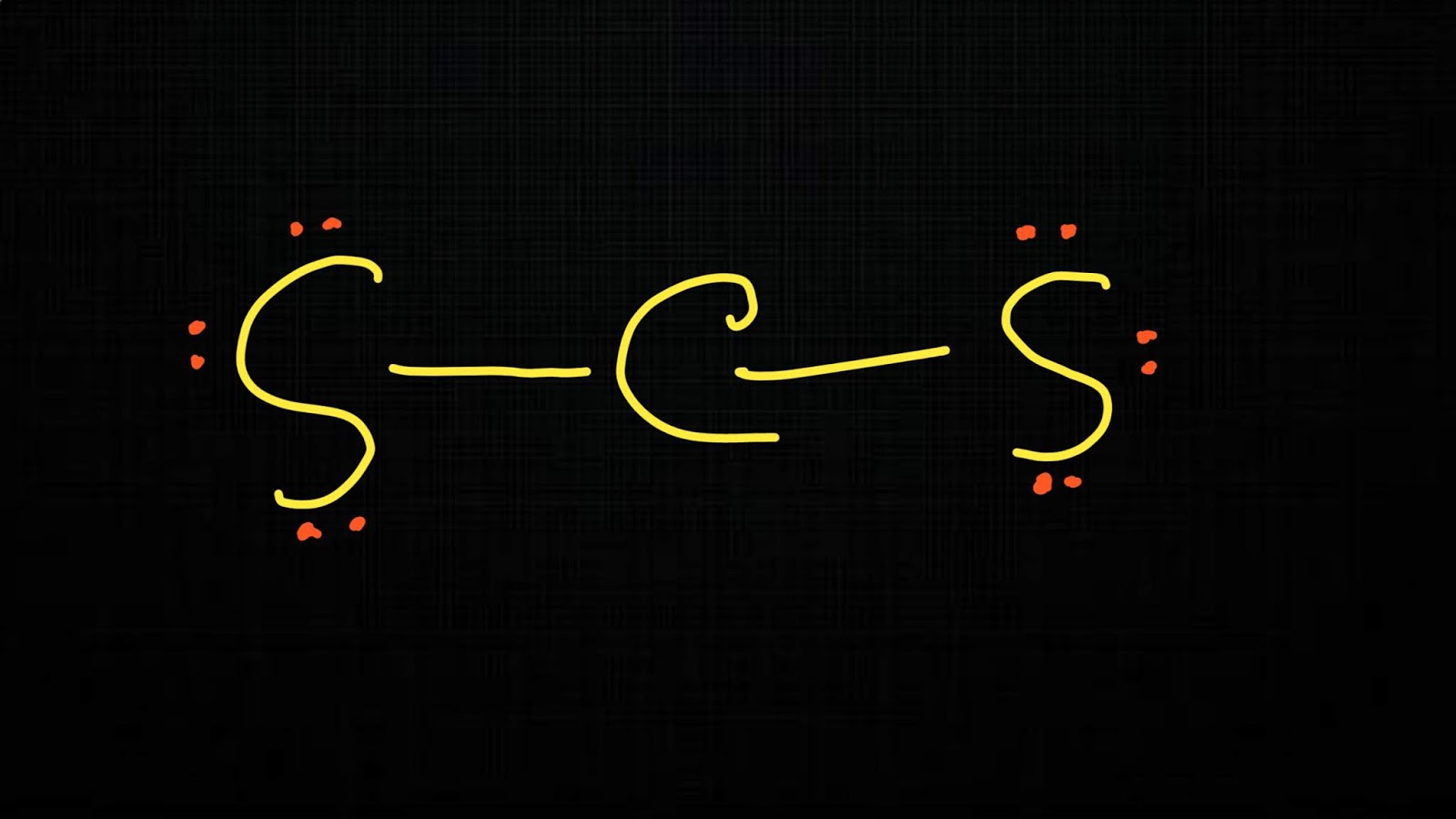
For the CS2 Lewis structure, calculate the total number of valence electrons for the CS2 molecule. After determining how many valence electrons there are in CS2, place them around the central atom to complete the octets. There are 16 valence electrons for the CS2 Lewis structure. Carbon is the least electronegative atom and goes in the center.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. The electron dot diagram of NH 3 is as follows: Exercise 12.4. 2. Use a Lewis electron dot diagram to show the covalent bonding in PCl 3. Answer.

What Is the Best Lewis Structure for Cs2
#1 First draw a rough sketch First, determine the total number of valence electrons Periodic table In the periodic table, carbon lies in group 14, and sulfur lies in group 16. Hence, carbon has four valence electrons and sulfur has six valence electrons. Since CS 2 has one carbon atom and two sulfur atoms, so…

CS2 Lewis Structure Lewis Structure for CS2 (Carbon Disulfide)Draw
Added Jun 9, 2014 by WebTester in Chemistry This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
CS2 Lewis Structure Molecular Geometry Polarity Hybridization
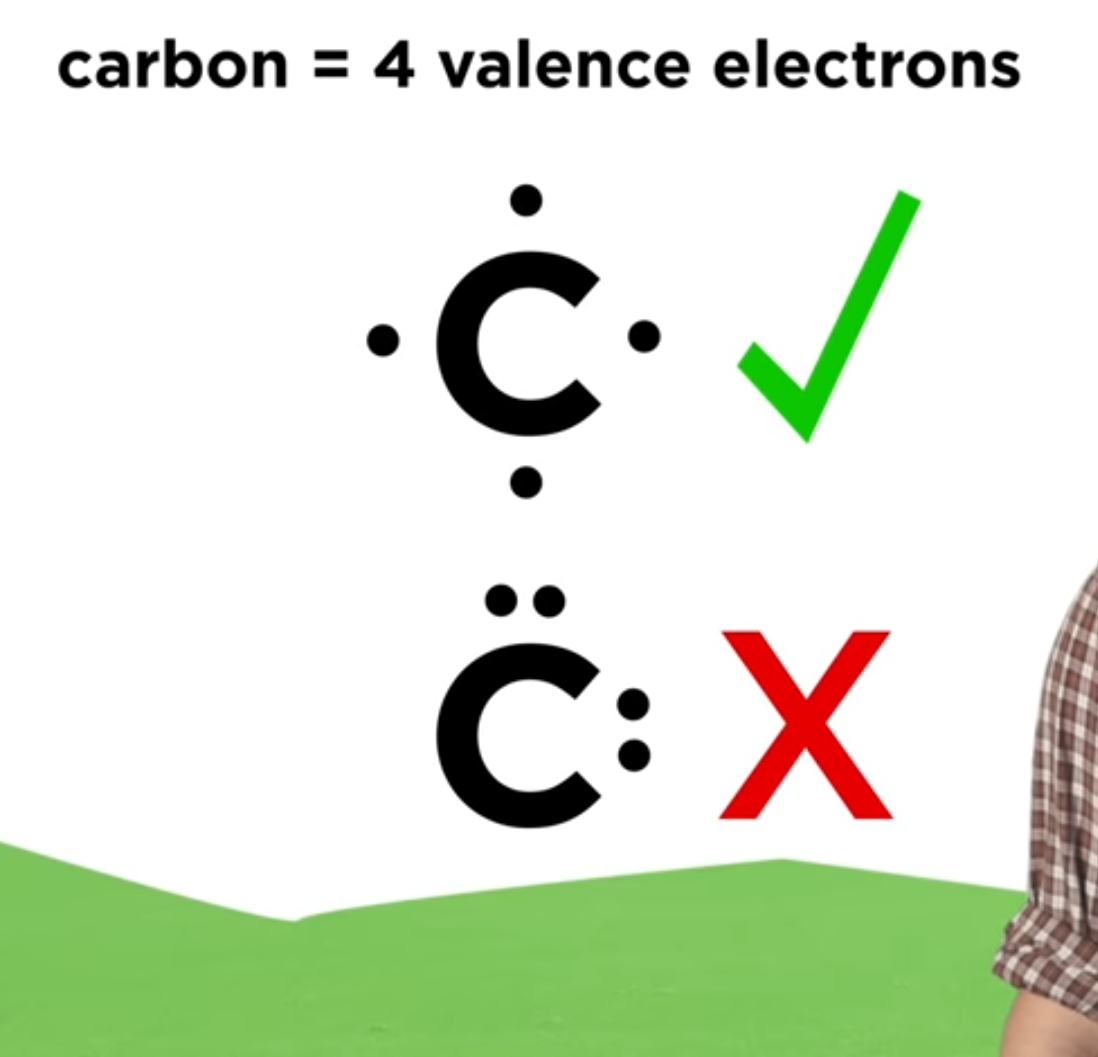
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table.
Lewis Dot Structure for CS2 Carbon disulfide YouTube
6 steps of CS2 Lewis structure: Step 1: Total number of valence electrons: Step 2: Determine the central metal atom: Step 3: Connect the atoms with dots: Step 4: Distribution of remaining valence electrons: Step 5: Check if all atoms have an octet: Step 6: Formal charge: Synthesis/Production: Direct Synthesis:

Cs2 Lewis Structure / Identify the geometry of if5 using vsepr theory
A step-by-step explanation of how to draw the CS2 Lewis Dot Structure (Carbon disulfide).For the CS2 structure use the periodic table to find the total numbe.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Watch on Steps of drawing CS2 lewis structure Step 1: Find the total valence electrons in CS2 molecule In order to find the total valence electrons in CS2 (carbon disulfide) molecule, first of all you should know the valence electrons present in carbon atom as well as sulfur atom.

CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape
CS2 Lewis Structure The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na - 1s 2 2s 2 2p 6 3s 1, Cl - 1s 2 2s 2 2p 6 3s 2 3p 5
CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
A quick explanation of the molecular geometry of CS2 including a description of the CS2 bond angles.Looking at the CS2 Lewis structure we can see that there.

How do you draw the Lewis structure of CS2 (Carbon disulfide) YouTube
To draw the Lewis structure for CS2, follow these steps: 1. Calculate the total number of valence electrons in CS2: Carbon (C) possesses 4 valence electrons, while sulfur (S) has 6 valence electrons. As there are two sulfur atoms, the total valence electrons in CS2 sum up to 16. 2. Identify the central atom, which is the least electronegative atom.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Learning Objectives By the end of this section, you will be able to: Write Lewis symbols for neutral atoms and ions Draw Lewis structures depicting the bonding in simple molecules Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions.

How to draw CS2 Lewis Structure? Science Education and Tutorials
A step-by-step explanation of how to draw the CS2 Lewis Dot Structure (Carbon disulfide).For the CS2 structure use the periodic table to find the total numbe.
CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
CS2 is an abbreviated form of Carbon Disulphide. This molecule has two Sulphur atoms and one Carbon atom. To understand the hybridization, molecular geometry and the polarity of this molecule it is essential to under its Lewis structure. Contents Lewis Structure Hybridization Molecular geometry Polarity Lewis Structure
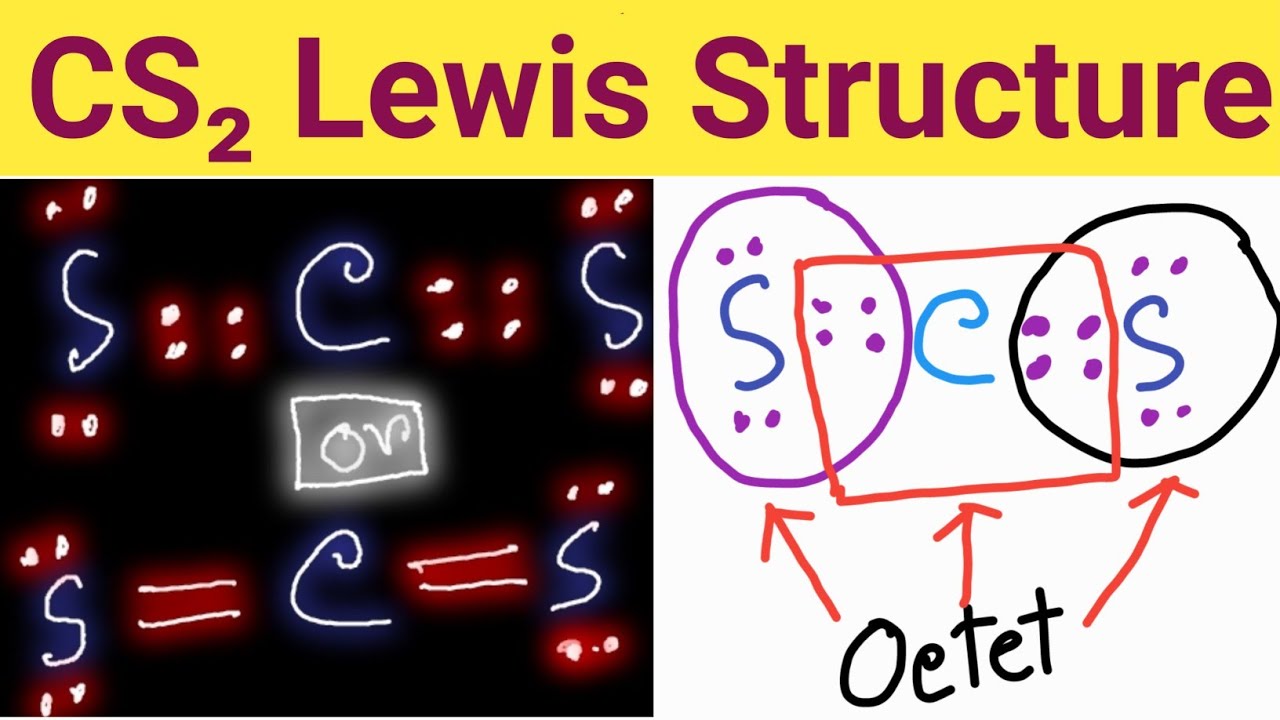
CS2 Lewis Structure Lewis Dot Structure for CS2 Carbon Disulfide
Step-1: CS2 Lewis dot Structure by counting valence electrons on the carbon atom Step-2: Lewis Structure of CS2 for counting valence electrons around the terminal sulfur atoms Step-3: Lewis dot Structure for CS2 generated from step-1 and step-2 How to calculate the formal charge on sulfur and carbon atoms in CS2 Lewis Structure?

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Drawing the Lewis Structure for CS 2 ( Sulfur Trioxide) CS 2 is sometimes used to fumigate railroad cars and grain elevators. CS 2 is named Carbon Disulfide. There are 16 valence electrons available for the Lewis structure for CS 2 . Video: Drawing the Lewis Structure for CS2. It is helpful if you:
